Background and Significance:
Although allogeneic hematopoietic cell transplantation (alloHCT) is a potentially curative therapy for patients with high-risk acute myeloid leukemia (AML), relapse occurs in 30% to 40% of alloHCT recipients and is associated with a 2-year post-relapse survival rate of less than 20%. Chimeric antigen receptor (CAR) T cells targeting tumor-associated antigens have demonstrated efficacy in relapsed/refractory (R/R) acute lymphoblastic leukemia and other hematologic malignancies, but there is limited clinical experience with CAR T cell therapies for AML. Current challenges for autologous AML CAR T cell therapy include the inability to harvest adequate numbers of functional, undifferentiated T cells as CAR T cell starting material. In addition, the potential presence of AML blasts in an autologous cell collection can interfere with T cell proliferation and function during the manufacturing process. To circumvent these impediments in patients with R/R AML after alloHCT, VCAR33 represents a CD33-directed CAR T cell product generated from lymphocytes from each patient's prior allogeneic stem cell donor. CAR T cell manufacture from HLA-matched donors will likely not only eliminate delays and failures in production due to lymphopenia but also improve T cell function by using healthy starting material.
CD33 is a preferential target for AML CAR T cell therapy as it is expressed on the majority (>80%) of AML blasts and because prior clinical experience with gemtuzumab ozogamicin (GO; tradename: Mylotarg TM) has demonstrated the safety and efficacy of targeting CD33.
Study Design and Methods:
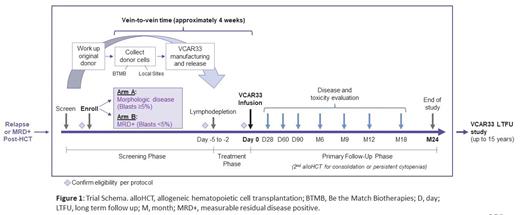
Study NCTxxxxxxxx* is a multi-center phase 1/2 study evaluating the safety and preliminary efficacy of VCAR33, donor-derived allogeneic CAR T cells targeting CD33 (Figure 1). The CAR construct used for the manufacture of VCAR33 contains a lintuzumab-derived binding domain and a CD28 co-stimulatory domain. Key inclusion criteria include adult patients with relapsed or measurable residual disease positive (MRD+) AML after undergoing alloHCT with an 8/8 HLA-matched related or unrelated donor. Patients who have undergone alloHCT with trem-cel, a CD33-deleted allogeneic stem cell product intended to facilitate the post-HCT treatment with CD33-directed therapies by reducing on-target off-tumor hematotoxicity, may also participate. Patients will be assigned to one of two study arms based on their AML disease burden: Arm A for morphologic disease with ≥ 5% blasts in the bone marrow, or Arm B for MRD+ defined as < 5% blasts in the bone marrow with ≥ 0.1% CD33+ leukemia cells by flow cytometry. The patient's prior stem cell donor undergoes apheresis for collection of mononuclear cells, which are used as starting material for VCAR33 manufacturing. Patients will receive lymphodepletion on days -5 to -2 with fludarabine (total 120 mg/m 2) and cyclophosphamide (total 1000 mg/m 2) followed by infusion of VCAR33 on Day 0. Each study arm will enroll and escalate independently according to a standard 3+3 trial design, starting at 1 x 10 6 CAR T cells/kg for Dose Level 1. The dose limiting toxicity monitoring period is 28 days, at which time patients will undergo evaluation of disease response. Secondary endpoints include incidence of cytokine release syndrome, graft-vs-host disease, and clinical response. Exploratory endpoints aim to correlate VCAR33 properties with clinical response by characterizing T cell phenotypes within the VCAR33 product and by examining VCAR33 expansion and persistence after infusion. Follow up on trial will continue for up to 2 years after infusion followed by long term monitoring for up to 15 years. Patients may undergo second alloHCT for consolidation of remission or for rescue of prolonged cytopenia due to on-target off-tumor effects of VCAR33.
*NCT number pending. Clinical trial information submitted to CT.gov on July 19, 2023.
Disclosures
Shah:Lentigen: Research Funding; CARGO: Consultancy; VOR: Consultancy, Research Funding; Immunoadoptive Cell Therapy Private Limited: Consultancy, Other: Scientific Advisory Board. DiPersio:Rivervest: Consultancy; Macrogenics: Research Funding; WUGEN: Current holder of stock options in a privately-held company, Other: Ownership Investment, Patents & Royalties, Research Funding; Magenta: Current holder of stock options in a privately-held company, Other: Ownership Investment, Patents & Royalties; Bioline: Consultancy; Vertex: Consultancy. Koura:BMS: Consultancy, Research Funding. Muffly:adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; orca bio: Research Funding; bms: Research Funding; jasper: Research Funding; amgen: Consultancy; pfizer: Consultancy; kite: Consultancy, Honoraria, Research Funding; autolus: Consultancy; astellas: Consultancy, Research Funding. Narayan:Novartis: Other: Research funding to institution; Sanofi: Other: Spouse employment . Nath:Vor Biopharma: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal